Experiment conducted on January 7, 2025
This lab journal serves as a personal record of experimental work and learning experiences. Some experimental details, procedures, and results have been simplified or omitted. This content is intended for personal documentation purposes only and should not be used as a reference for scientific work or experimental procedures.Overview
This experiment focused on determining ethylene glycol content in hydrolysed polyester samples using gas chromatography with an internal standard method. Using a Thermo Scientific FOCUS GC with a CP-Wax 57CB column and FID, we aimed to quantify ethylene glycol using 2-butanol as an internal standard. Based on typical hydrolysis conditions, we hypothesised an efficiency of approximately 50% (0.438 M for complete hydrolysis of 200 g polyester in 1 L solution).
Method
A stock solution was prepared from pure ethylene glycol (density 1.12 kg/L, Mw 62.07 g/mol, 18.05 M) and diluted tenfold to reach 1.805 M. Calibration standards were prepared according to Table 1:
Table 1: Calibration series preparation using 1.805 M
ethylene glycol stock solution and 2-butanol as internal standard
| Concentration (M) | Volume of Stock Solution (mL) | Volume of 2-Butanol (mL) | Total Volume (mL) |
| 0.072 | 2 | 2 | 50 |
| 0.181 | 5 | 2 | 50 |
| 0.361 | 10 | 2 | 50 |
| 0.542 | 15 | 2 | 50 |
| 0.722 | 20 | 2 | 50 |
| 1.444 | 40 | 2 | 50 |
The hydrolysed samples were prepared by combining 2 mL of sample with 0.2 mL of 2-butanol and diluting to 5 mL. GC analysis was performed with the following parameters: inlet temperature 250°C, split ratio 100, carrier gas flow 1.0 mL/min, and FID temperature 250°C. The oven program started at 60°C (3-min hold), ramped to 220°C at 40°C/min (4-min hold), with total run time of 11 minutes.
Results
1. Peak Identification
Proper identification of retention times for each compound is critical before quantitative analysis. Three compounds – pure ethylene glycol, 2-butanol (internal standard), and acetone (cleaning solvent) – were injected individually into the GC under identical operating operating conditions. The retention times were recorded: acetone elutes first (5.4 min), followed by 2-butanol (6.5 min), and finally ethylene glycol (9.8 min).
2. Quantification Method 1: Response Factor
A response factor approach was first applied to determine the ethylene glycol (EG) concentration in the hydrolysed sample. In this method, standard #3 (0.542 M EG) was used to calculate the RF, based on the ratio of the peak areas of ethylene glycol (EG) to the internal standard 2-butanol (IS). Because the same 2-butanol proportion was used in both standards and samples, its concentration was treated as 1.0 arbitrary unit. Using areas from standard #3 (0.542 M EG, AEG = 111,686, AIS = 652,397), the RF obtained was 0.316.
\[\text{RF} = \frac{\frac{A_{\text{EG}}}{C_{\text{EG}}}}{\frac{A_{\text{IS}}}{C_{\text{IS}}}} = \frac{\frac{111,686}{0.542}}{\frac{652,397}{1}} = \frac{206,062.73}{652,397} = 0.316\]Once the RF was known, the concentration in each sample was calculated by rearranging the equation. The average concentration of ethylene glycol across the hydrolysed textile samples was 0.423 M, with a standard deviation of 0.112, and coefficient of variation of 26.4%.
3. Quantification Method 2: Calibration Curve
The second method involved constructing a calibration curve by plotting the ratio of EG peak area to 2-butanol peak area (AEG/AIS) against the known ethylene glycol concentrations in four standards. Linear regression of these data provided a slope of 0.3847 and an intercept of -0.0169, with R2 = 0.9762. (Figure 1)
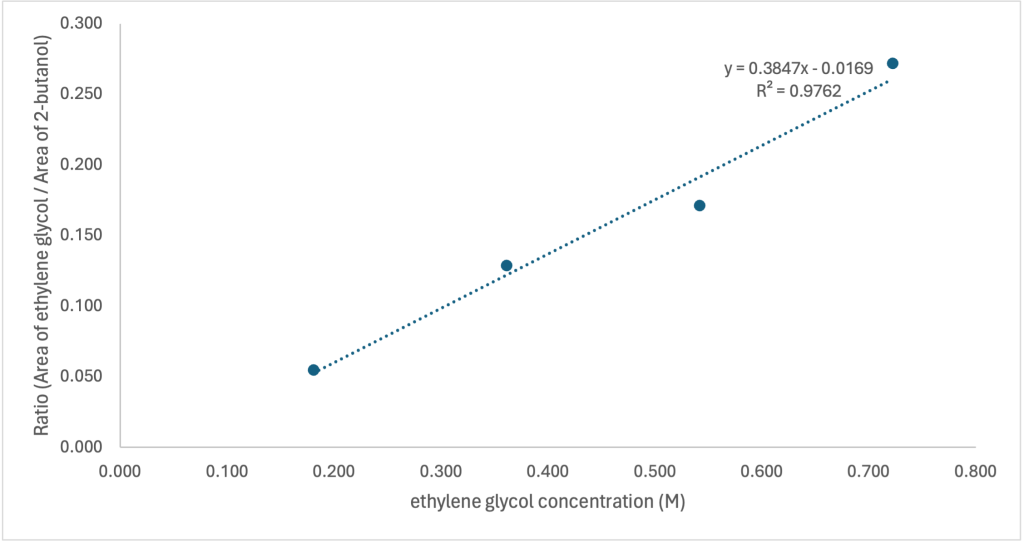
Figure 1: Calibration curve for ethylene glycol quantification showing the relationship between ethylene glycol concentration (M) and the ratio of peak areas (AEG/AIS)
Using the calibration curve, the ethylene glycol concentrations in the hydrolysed samples were calculated. The average concentration across all samples was determined to be 0.391 M, with a standard deviation of 0.092 and a coefficient of variation of 23.4%.
Leave a Reply