Experiment conducted on September 23, 2024
This lab journal serves as a personal record of experimental work and learning experiences. Some experimental details, procedures, and results have been simplified or omitted. This content is intended for personal documentation purposes only and should not be used as a reference for scientific work or experimental procedures.Overview
This experiment explored the application of High-Performance Liquid Chromatography (HPLC) for quantitative analysis of pharmaceutical compounds. Using an Agilent 1100 Series HPLC with VWD detector and reversed-phase C18 column, we aimed to separate and quantify paracetamol and caffeine in a mixture.
Method
Calibration standards were prepared using stock solutions of paracetamol and caffeine (1000 mg/L) according to Table 1:
Table 1: Pipetting schedule for the calibration series with paracetamol and caffeine standard solution in 100 mL volumetric flasks
| Standard | Desired concentration of Paracetamol (ug/mL) | Desired concentration of Caffeine (ug/mL) | Volume of Paracetamol 1mg/mL standard solution (mL) | Volume of Caffeine 1mg/mL standard solution (mL) |
| 1 | 25 | 10 | 2.5 | 1.0 |
| 2 | 50 | 20 | 5.0 | 2.0 |
| 3 | 100 | 30 | 10.0 | 3.0 |
| 4 | 150 | 40 | 15.0 | 4.0 |
| 5 | 200 | 50 | 20.0 | 5.0 |
Sample preparation involved 20-fold dilution in triplicate. The analysis was performed after system stabilization and method verification using SST (System Suitability Test). Individual standards were analysed to determine retention times before sample analysis.
Results
1. Elution Order
The separate diluted paracetamol and caffeine standard solutions were measured, and their elution times were recorded. The retention times for the standards were as follows:
- Paracetamol Standard: 2.638 minutes
- Caffeine Standard: 4.619 minutes
These measurements confirm that paracetamol elutes before caffeine under the experimental conditions employed, as expected. Based on their structures, paracetamol (acetaminophen) is more polar due to its hydroxyl and amide groups, while caffeine is less polar with multiple methyl groups. In reversed-phase HPLC with a non-polar C18 stationary phase and a polar mobile phase, more polar compounds like paracetamol elute first because they have lower affinity for the stationary phase.
2. Calibration Curves
Calibration curves were constructed for both paracetamol and caffeine. Two separate calibration plots were generated: one plotting peak area against concentration (Figure 1) and the other plotting peak height against concentration. (Figure 2)
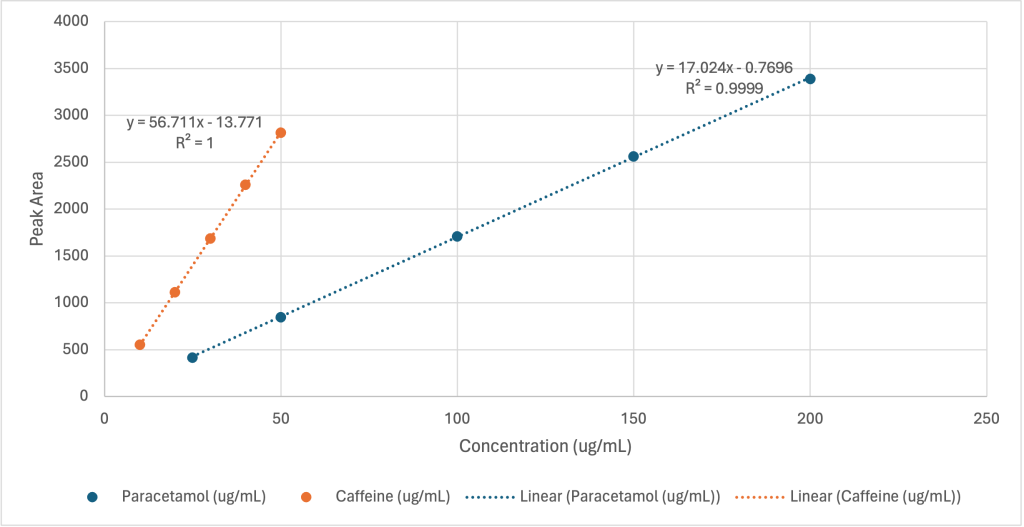
Figure 1: Calibration curves based on peak area. Peak area versus concentration for paracetamol
(blue) and caffeine (orange). R2 = 0.9999 for paracetamol and R2 = 1.0000 for caffeine.
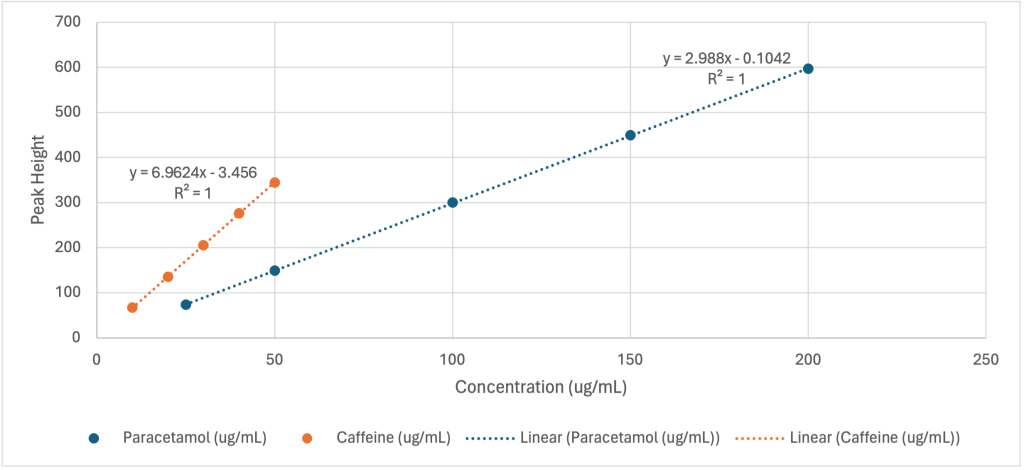
Figure 2: Calibration curves based on peak height. Peak height versus concentration for paracetamol (blue) an caffeine (orange). R2 = 1.0000 for both paracetamol and caffeine.
3. Quantification of Sample Concentrations
Using the calibration curves, the concentrations of paracetamol and caffeine in the triplicate diluted samples were calculated. Considering the 20-fold dilution factor, the original sample concentrations are summarised in Table 2.
Table 2: Concentrations of Paracetamol and Caffeine in Sample based on Peak Area and Peak Height
| Method | Compound | Mean Concentration (µg/mL) | Standard Deviation (µg/mL) | Coefficient of Variation (%) |
| Peak Area | Paracetamol | 461.1 | 135.5 | 29.4% |
| Caffeine | 863.1 | 11.1 | 1.3% | |
| Peak Height | Paracetamol | 390.3 | 7.7 | 2.0% |
| Caffeine | 859.4 | 11.3 | 1.3% |
The concentration of paracetamol was determined to be 461.1 ± 135.5 µg/mL based on peak area and 390.3 ± 7.7 µg/mL based on peak height. The concentration of caffeine was determined to be 863.1 ± 11.1 µg/mL based on peak area and 859.4 ± 11.3 µg/mL based on peak height.
The concentrations determined via peak height demonstrated higher precision, as evidenced by the significantly lower coefficients of variation (CV) for both analytes, particularly for paracetamol (CV = 2.0%) compared to the peak area method (CV = 29.4%).
Leave a Reply